Introduction
Secondary structures are elements of great importance in structural biology, biochemistry and bioinformatics. They are broadly composed of two repetitive structures namely α-helices and β-sheets, apart from turns, and the rest is associated to coil. These repetitive secondary structures have specific and conserved biophysical and geometric properties.
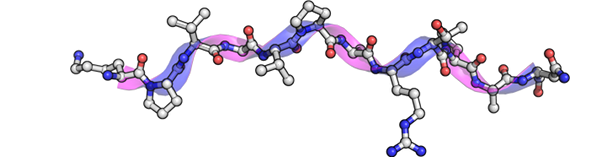
PolyProline II (PPII) helix is yet another interesting repetitive structure which is less frequent and not usually associated with stabilizing interactions. Recent studies have shown that PPII frequency is higher than expected, and they could have an important role in protein – protein interactions.
A major factor that limits the study of PPII is that its assignment cannot be carried out easily. The purpose of PolyprOnline database and server is to identify and analyse PPII in protein structures and propose a user friendly interface to encourage study of PPII.
You can start a search in PolyPrOnline database in Search section. For further details about the different functionalities please go to How to section.
When using this website and database, please cite: Chebrek R, Leonard S, de Brevern AG, Gelly J-C (2014) PolyprOnline: polyproline helix II and secondary structure assignment database ; DATABASE (OXFORD) ; Nov 7;2014 [pmid:25380779].
Database statistics
The database contains 24761 protein structures.
Database is updated every 6 months.
Complete database update is performed from a PDB list generated by PISCES web service using criterions of maximal mutual sequence identity of 90% between sequence proteins,
resolution <3.0 and R <1.0.
(Wang, G., Dunbrack, R.L., Jr. (2005) PISCES: recent improvements to a PDB sequence culling server. Nucleic acids research, 33, W94-98.).
Last update: 2023-02-20 19:19:21