The CSB motif
|
|
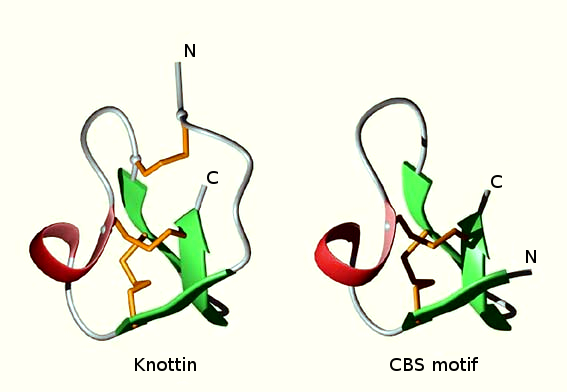
It has been noticed that knottins, that contain at least
three disulfide bridges, are built from a possibly ancestral
two-disulfide elementary motif.
One such motif has been termed the CSB (Cystine Stabilized β-sheet)
motif [Heitz
et al, 1999]. |
 |
|
The minimum peptide, known as
Min-23, correponding to the isolated CSB motif has been shown
to be an autonomous folding unit with a significant thermal stability
(Tm = 100 °C) [Heitz
et al, 1999]. |
|
The Min-23 peptide has been used as a structural motif
to build chimeras of Angiotensin-Converting Enzyme in which the
JuxtaMembrane stalk is replaced by Min-23 [Schwager
et al., 2001]. It is shown that, in contrast to native JuxtaMembrane
stalk or to chimere using EGF, the Min-23 chimere is not cleaved
inside the motif but rather next to it. |
The Min-23 peptide has been used as a small and stable structural scaffold
to obtain novel binding specificities by selection from a display library
[Souriau
et al., 2005]. It is shown that, in contrast to native JuxtaMembrane
stalk or to chimere using EGF, the Min-23 chimere is not cleaved
inside the motif but rather next to it. |
The DDH motif
|
A slightly different description has also been given and termed
the DDH (Disulfide-Directed β-Hairpin) motif [Wang
et al., 2000].
The DDH motif contains a β-hairpin instead of a triple-stranded
β-sheet. |


